Crystal Lake, Illinois, May 8, 2020 – AptarGroup, Inc. (NYSE: ATR), a global leader in drug delivery, consumer product dispensing and active packaging solutions, is seeking Emergency Use Authorization (EUA) from the German Federal Institute for Drugs and Medical Devices (BfArM) for a solution that allows for easy disinfecting of FFP2 filtering facepiece respirators (FFP2 masks). The FFP2 masks are desperately needed by healthcare personnel due to the global shortage of disposable masks during the COVID-19 pandemic.
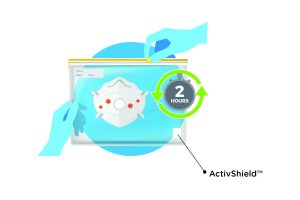
In this simple disinfecting process, the FFP2 mask and a small strip of Aptar’s ActivShield™ technology are placed inside any commonly available one-gallon plastic bag. The strip releases a controlled amount of chlorine dioxide inside the sealed bag to decontaminate the FFP2 mask. The process takes only two hours until the mask is ready to wear again. It can be performed on-site at the local hospital where the mask is being used. The EUA submission in Germany is part of Aptar’s strategy to expand global access to FFP2 respirators allowing for the decontamination and reuse of masks for ten cycles. This innovative patent pending technology can efficiently and effectively disinfect FFP2 masks used by healthcare workers and first responders.
Aptar has submitted its safety and effectiveness data to the FDA for EUA review. The company is working to provide approximately four million ActivShield strips per week and is working to expand its production capacity with the intent to deliver ten million per week by the end of April. “We are extremely encouraged by the promising data generated so far and are eager to deliver this technology to the front line and support the fight against the pandemic,” said John Belfance, President of Aptar CSP Technologies. If the FDA approves the EUA, then ActivShield™ will immediately become available for this important use.
The German EUA BfArM submission follows Aptar’s U.S. FDA Emergency Use Authorization submission in early April. Germany is thus the first country in the EU where an application to this effect has been submitted to the authorities.
“We are proud to continue to support the fight against the COVID-19 pandemic by furthering our efforts to bring this innovative technology to the global health care community. We have a long-standing presence in Germany and we will continue to support the communities where we live and work,” said Stephan Tanda, Aptar President and CEO. “Our technology provides a unique, simple, and effective way to help solve the critical problem of PPE shortages we’re currently facing. We look forward to working with the U.S. FDA and BfArM to bring ActivShield™ to market and fulfill the ongoing unmet need.”
Chlorine dioxide has been widely used as a disinfectant in different industries, including the paper industry, drinking water treatment, food processing, and medical equipment. Aptar’s delivery mechanism uses the disinfecting properties of chlorine dioxide in a controlled sustained release within a contained volume.
Studies on the ActivShield™ system show the treatment to be 99.999% effective against viruses. Detailed data on the effectiveness and safety of the ActivShield™ system is available at www.aptaractivshield.com.
About Aptar
Aptar is a global leader in the design and manufacturing of a broad range of drug delivery, consumer product dispensing and active packaging solutions. Aptar uses insights, design, engineering and science to create dosing, dispensing and protective packaging technologies for the world’s leading brands, in turn making a meaningful difference in the lives, looks, health and homes of millions of people around the world. Aptar’s innovative solutions and services serve a variety of end markets including pharmaceutical, beauty, personal care, home, food and beverage. The company is headquartered in Crystal Lake, Illinois and has 14,000 dedicated employees in 20 countries. For more information, visit www.aptar.com.
This press release contains forward-looking statements. Words such as “promising” or “working” and other similar expressions or future or conditional verbs such as “will” or “could” are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: economic conditions worldwide; uncertainties related to the timing or outcome of product development; the regulatory environment; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the Securities and Exchange Commission, including the discussion under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Form 10-Ks and Form 10-Qs. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
U.S. Product Contact:
John Belfance
john.belfance@aptar.com
518-469-3358
Europe Contact:
Carolyn Penot
carolyn.penot@aptar.com
+33 6 37 36 76 84
Corporate Contact:
Matt DellaMaria
matt.dellamaria@aptar.com
815-479-5530