Crystal Lake, Illinois, October 22, 2024 – AptarGroup, Inc. (NYSE: ATR), a global leader in drug and consumer product dosing, dispensing and protection technologies, announced that it was awarded a contract from the U.S. Federal Government to advance development of its ActivShield™ technology. This innovative solution sterilizes medical devices and instruments without the need for a power source, making it a versatile solution for numerous environments, including rural areas, military settings and healthcare facilities with limited or no current sterilization capability.
ActivShield™ technology leverages Aptar CSP Technologies’ 20+ years of material science expertise and is built upon the company’s proven 3-Phase Activ-Polymer™ platform, a highly engineered active material science solution trusted by global brands to protect sensitive drug products, medical devices, drug delivery systems, and probiotics.
ActivShield™ technology is a portable, novel sterilization modality that does not require conventional infrastructure, power, or extensive training, and more importantly, does not present the health risk associated with the use of Ethelyne Oxide (EtO). ActivShield™ uses a highly engineered Activ-Film™ material that emits a controlled amount of chlorine dioxide gas to sterilize a wide range of medical devices and instruments.
“As an innovation leader in pharma dosing, dispensing and protection technologies, we are proud to work with the U.S. Government to advance our ActivShield™ solution for the sterilization of medical devices and instruments in locations where a power source may not be available, further enhancing its versatility,” said Stephan B. Tanda, Aptar President and CEO.
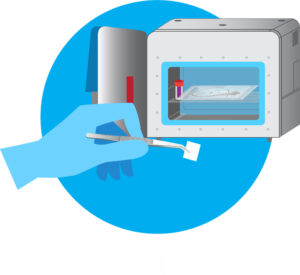
Figure 1: ActivShield™ film ready to be placed in
chamber for sterilization (chamber not required)
Aptar’s technology can be custom engineered to meet the needs of specific devices and conditions. Its stability throughout shelf life and compact size (Figure 1) makes it easy to stockpile, and all materials are manufactured in the United States.
ActivShield™ technology is well-positioned to fill unmet sterilization needs in remote or emergency response civilian settings, which could significantly reduce infection risks stemming from inadequately sterilized medical devices and instruments. Additionally, the technology could reduce reliance on expensive and unsafe sterilization techniques such as EtO, which has come under recent scrutiny by the EPA for toxic emissions.
For the military, ActivShield™ can help wounded service members, particularly those in far-forward environments, have access to sterilized instruments during critical pre-hospitalization periods, following severe injuries.
John Belfance, president of Aptar CSP Technologies, stated, “ActivShield™ technology is a breakthrough in material science technology that has the potential to dramatically expand reliable instrument sterilization for challenging environments, simplifying processes and helping to save lives. We are grateful for the government’s recognition, which supports the potential of this new technology, and look forward to bringing ActivShield™ technology to the forefront of sterilization techniques.”
The five-year contract is valued at approximately $4.8 million.
* This work is supported by the US Army Medical Research and Development Command under Contract No. HT9425-24-C-0078. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
About Aptar
Aptar is a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar serves a number of attractive end markets including pharmaceutical, beauty, food, beverage, personal care and home care. Aptar CSP Technologies leverages its active material science expertise to transform ideas into market opportunities, accelerate and de-risk the product development process, and provide complete solutions that improve consumers’ and patients’ lives. The company offers a complete set of services from concept ideation, to design and engineering, to product development, global production, quality control, and regulatory support that results in expedited speed-to-market. For more information, please visit www.csptechnologies.com and www.aptar.com.
About Aptar CSP Technologies
Aptar CSP Technologies is part of AptarGroup, Inc., a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar CSP Technologies leverages its active material science expertise to transform ideas into market opportunities, accelerate and de-risk the product development process, and provide complete solutions that improve consumers’ and patients’ lives. The company offers a complete set of services from concept ideation, to design and engineering, to product development, global production, quality control, and regulatory support that results in expedited speed-to-market. For more information, please visit www.csptechnologies.com and www.aptar.com.
This press release contains forward-looking statements, including the potential outcomes and the value of the contract for the development of the ActivShield™ technology. Forward-looking statements generally can be identified by the fact that they do not relate strictly to historical or current facts and by use of words such as “expects,” “anticipates,” “believes,” “estimates,” “future,” “potential,” “continues” and other similar expressions or future or conditional verbs such as “will,” “should,” “would” and “could” are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results or other events may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: the successful integration of acquisitions; the regulatory environment; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the Securities and Exchange Commission, including the discussion under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Form 10-K and Forms 10-Q. We undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as otherwise required by law.
